Please click on this link and watch the video:
https://www.prostate.org.au/news-media/news-archive/news-archive-2017/australian-first-trial-set-to-revolutionalise-treatment-of-advanced-prostate-cancer/
Please read this carefully, it reports on a huge advance in therapy! A potential cure from metastatic PCa – normally fatal. A pity it is not described as such. Amazing good news!
Monday, July 2, 2018
News & Perspective Drugs & Diseases CME & Education Academy Video New
News > Medscape Medical News > Oncology News
‘Image of the Year’ Captures Dramatic Cancer Responses
Nick Mulcahy
June 28, 2018
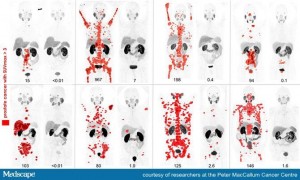
A set of dramatic before-and-after images of eight men with widespread metastatic prostate cancer who underwent treatment with cutting-edge technology has been awarded the 2018 Image of the Year from the Society of Nuclear Medicine and Molecular Imaging (SNMMI).
Peter MacCallum
The honor went to a group of prostate cancer researchers led by Prof Michael Hofman, MD, at the Peter MacCallum Cancer Centre in Melbourne, Australia.
The Image of the Year was picked from more than 2200 imaged-oriented abstracts submitted during the recent SNMMI annual meeting in Philadelphia, Pennsylvania, and was voted on by reviewers and the society leadership.
The images of eight patients who demonstrated exceptional response to treatment were produced from the Australian center’s phase 2 prospective trial of 30 patients with metastatic prostate cancer that had become castration resistant and thus no longer responded to standard hormonal therapy.
For the trial, the researchers used gallium-68 prostate-specific membrane antigen–11 (PSMA11) positron-emission tomography (PET) imaging to screen men who had exhausted conventional therapies, which included androgen deprivation therapy, arbiraterone, and chemotherapy.
For their trial, they selected men with high PSMA expression.
The patients went on to receive treatment with Lutetium-177 PSMA617 (LuPSMA), a radiolabeled small molecule that binds with high affinity to PSMA and delivers a therapeutic dose of radiation to cancer cells.
As part of the phase 2 trial, the extent of tumor spread was imaged at baseline and 3 months after treatment using PSMA PET.
The images show the recession of metastatic disease after treatment. The researchers note that this was accompanied by significant improvements in pain scores at all time points. Median PSA progression-free survival was 7.6 months (95% confidence interval [CI], 6.4 – 9.0), and median overall survival was 13.5 months (95% CI, 10.4 – 22.7).
“These compelling results indicate the need for randomised trials comparing LuPSMA to existing standard-of-care,” write the Australian team in their meeting abstract.
Hofman believes that nuclear medicine will join other specialties as a key contributor to the care of these patients.
“These images tell a story about exceptional responses observed in patients who had progressed after standard therapies. They match striking improvements in patient quality of life. With further research, we look forward to seeing nuclear medicine evolve as a key specialty and standard of care in cancer management,” he said in a statement.
Umar Mahmood, MD, PhD, chair of the SNMMI Scientific Program Committee, commented: “This phase 2I prospective study by Michael Hofman and colleagues clearly shows the benefit to men with castrate-resistant prostate cancer treated with a beta emitter targeting PSMA when their tumors expressed PSMA. It is gratifying to see the benefit of the approach both rigorously and objectively demonstrated in this trial, in terms of improvement in disease burden and improvement in the pain severity.”
Patients Selection Optimized Outcomes
The presentation of data at the SNMMI meeting is an update of earlier presentations about the new therapy, including one at the 2017 European Society of Medical Oncology annual meeting.
At that time, Prof Johann de Bono, MD, of the Royal Marsden Hospital and the Institute of Cancer Research in London, United Kingdom, commented to Medscape Medical News that the early trial results were “very encouraging.”
De Bono also observed that patient selection optimized the outcomes seen with the treatment, which involves use of a radioimmunoconjugate. “They selected patients with high PSMA expression or anti-PSMA antibody uptake with diagnostic pretreatment scans, and they did not recruit patients who had discordance with high choline PET uptake and low PSMA PET uptake,” he said.